X-linked ichthyosis
| X-linked ichthyosis | |
|---|---|
| Other names | Steroid sulfatase deficiency, X-linked recessive ichthyosis[1] |
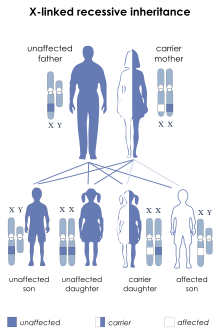 | |
| X-linked recessive inheritance: Affected boys may inherit a deletion or mutation of the STS gene from their mothers | |
| Specialty | Medical genetics |
X-linked ichthyosis (abbreviated XLI) is a skin condition caused by the hereditary deficiency of the steroid sulfatase (STS) enzyme that affects 1 in 2000 to 1 in 6000 males.[2] XLI manifests with dry, scaly skin[3] and is due to deletions[4][5] or mutations[6] in the STS gene. XLI can also occur in the context of larger deletions causing contiguous gene syndromes.[4] Treatment is largely aimed at alleviating the skin symptoms.[7] The term is from the Ancient Greek 'ichthys' meaning 'fish'.
Signs and symptoms
[edit]
The major symptoms of XLI include scaling of the skin, particularly on the neck, trunk, and lower extremities. The extensor surfaces are typically the most severely affected areas. The >4 mm diameter scales adhere to the underlying skin and can be dark brown or gray in color. Symptoms may subside during the summer.[2]
Associated medical conditions
[edit]Aside from the skin scaling, XLI is not typically associated with other major medical problems.[8] Atrial fibrillation or atrial flutter may affect up to 1 in 10 males with XLI.[9] Heart rhythm abnormalities in individuals with XLI tend to co-occur with disorders of the gastrointestinal tract, and are likely to result from steroid sulfatase deficiency.[10] Cardiac arrhythmia in XLI may be related to abnormal development of the interventricular septum or interatrial septum.[11] Corneal opacities may be present but do not affect vision. Cryptorchidism is reported in some individuals.[2] Individuals with XLI appear at increased risk of neurodevelopmental disorders such as autism and attention deficit hyperactivity disorder and some affected individuals exhibit mood problems [12] Mood problems in XLI appear to be most influenced by stigma or bullying associated with the skin condition, and by difficulties with treating the skin condition.[13] XLI is associated with mild-moderate impairments in memory which appear to be independent of effects on mood.[14] Blood-clotting abnormalities may occur more frequently in males with XLI and female carriers.[15] Individuals with XLI can exhibit intellectual disability, although this is thought to be due to deletions encompassing neighboring genes (e.g. VCX) in addition to STS.[16] The skin and medical conditions associated with XLI are likely to be due to perturbed basement membrane function and abnormal interactions with the extracellular matrix.[17] Knockdown of STS gene expression in human skin cell cultures affects pathways associated with skin function, brain and heart development, and blood-clotting that may be relevant for explaining the skin condition and increased likelihood of ADHD/autism, cardiac arrhythmias and disorders of hemostasis in XLI[18]
Female carriers generally do not experience any of these problems but can have difficulty during childbirth, as the STS expressed in the placenta plays a role in normal labor.[19] Female carriers may also be at slightly increased risk of developing mental health problems following childbirth [20] For these reasons carriers should ensure their obstetrician is aware of the condition.[citation needed]
Genetics
[edit]
The STS gene is located on the X chromosome at band Xp22.3. Thus, the syndrome is an X-linked condition, and it affects males and females differently. The 23rd pair of chromosomes is typically termed the "sex chromosomes". Females have two X chromosomes and males have one X and one Y chromosome. Therefore, in normal individuals, males carry a single copy of the STS gene and females carry two copies. This gene partially escapes X-inactivation and females normally express higher amounts of the STS enzyme than males.[21]
XLI can occur through new deletions or mutations of the STS gene but is more commonly inherited from a carrier mother.[22] A hemizygous deletion or mutation of the STS gene in a male results in complete absence of enzyme activity, while a female carrier of a mutation or deletion is heterozygous and still has a normal copy of the STS gene. Female carriers of an STS deletion or mutation still express the STS enzyme, although with decreased enzyme activity.[23]
For this reason, XLI most commonly affects males, although individuals with numeric abnormalities of the sex chromosomes (45,X and 47,XXY) who also carry STS deletions or mutations would be exceptions to this rule.[citation needed]
In addition, a female could be affected if she were the offspring of an affected male and a carrier female and inherited a deletion or mutation of the STS gene on both X chromosomes.[citation needed]
Genetic counseling issues
[edit]Since the majority of cases appear to occur through transmission of an STS deletion from a carrier mother,[22] enzyme testing or DNA testing should be performed in the mother of any newly diagnosed simplex case (i.e. the first case in a family). In the case of an extended family with many affected individuals, carrier status can often be assigned based on pedigree analysis.
- Males with XLI will transmit the X chromosome harboring the STS deletion or mutation to each of his female offspring, who will therefore be an obligate carrier. However, all male offspring will be unaffected, since they receive their father's Y chromosome.[citation needed]
- Female carriers of an STS deletion or mutation have a 50% chance with each pregnancy of transmitting it to an offspring. Thus, each male offspring has a 50% chance of being affected by XLI, while each female offspring has a 50% chance of being a carrier for this condition. Any individual that inherits the mother's normal copy of the STS gene will be unaffected and will have an extremely low chance of having a child affected with this condition.[citation needed]
Due to random segregation of the chromosomes during gametogenesis, each pregnancy will be subject to the same probabilities, regardless of the number of previously affected or unaffected offspring. The above recurrence risks are based on the assumption that an affected male or carrier female will have children with an unaffected or non-carrier individual. The risks of having affected offspring would clearly increase in the case of a union between a male with XLI and a carrier female.[citation needed]
Physiology/biochemistry
[edit]
The STS enzyme (EC 3.1.6.2), also referred to as Arylsulfatase C, is expressed throughout the body, with highest expression in the skin, liver, lymph nodes, and placenta, and lower expression in breast tissue and brain[24] STS catalyzes the hydrolysis of sulfated steroids, such as estrone sulfate and dehydroepiandrosterone sulfate (DHEAS), to non-sulfated steroids estradiol and androstenediol, respectively.[25] Prenatally, the enzyme is involved in placental estrogen production.[26] The enzyme is also involved in adrenal steroid production as well as conversion of sulfated steroids in other tissues.[citation needed]
There seems to be a particularly important role for the enzyme in skin. Deficiency of the enzyme leads to the characteristic dry and scaly skin seen in ichthyosis. Recent research indicates that the skin abnormalities seen in XLI may be due to accumulation of cholesterol sulfate in the outer epidermis, leading to abnormal barrier function and corneocyte retention.[27]
Diagnosis
[edit]XLI can be suspected based on clinical findings, although symptoms can take varying amounts of time to become evident, from a few hours after birth, up to a year in milder cases. The diagnosis is usually made by a dermatologist, who also typically formulates the treatment plan (see below). STS enzyme deficiency is confirmed using a clinically available biochemical assay. Carrier detection can be performed in mothers of affected sons using this test (see Genetics, below).[23] Molecular testing for DNA deletions or mutations is also offered, and can be particularly useful in the evaluation of individuals with associated medical conditions (see below). Prenatal diagnosis is possible using either biochemical or molecular tests. However, the use of prenatal diagnosis for genetic conditions that are considered to be generally benign raises serious ethical considerations and requires detailed genetic counseling.[citation needed]
Treatment
[edit]Because XLI is caused by a gene mutation or deletion, there is no "cure." One of the aims of treatment is to reduce scaling by removing the excess, flaky scales, and keep the skin hydrated. This can be achieved using a variety of topical creams.[2][7] Other treatments involve
- Keratolytic agents such as Ammonium lactate (Lac-Hydrin) are used to facilitate the release of retained corneocytes.[citation needed]
- oral isotretinoin[citation needed]
- acitretin[28]
- The topical receptor-selective retinoid tazarotene [29]
Research is ongoing with regard to the use of gene therapy to treat XLI.[30] Timber Pharmaceuticals is planning on conducting a phase 3 trial of its investigational topical isotretinoin product in the second quarter of 2022 for the treatment of congential ichthyosis.[31]
History
[edit]In the 1960s, recessive x-linked ichthyosis was distinguished clinically from other ichthyoses.[32]: 486 [33]: 561
See also
[edit]References
[edit]- ^ Rapini, Ronald P.; Bolognia, Jean L.; Jorizzo, Joseph L. (2007). Dermatology: 2-Volume Set. St. Louis: Mosby. ISBN 978-1-4160-2999-1.
- ^ a b c d Carlo Gelmetti; Caputo, Ruggero (2002). Pediatric Dermatology and Dermatopathology: A Concise Atlas. T&F STM. p. 160. ISBN 978-1-84184-120-5.
- ^ Online Mendelian Inheritance in Man (OMIM): ICHTHYOSIS, X-LINKED - 308100
- ^ a b Ballabio A, Parenti G, Carrozzo R, et al. (1987). "Isolation and characterization of a steroid sulfatase cDNA clone: genomic deletions in patients with X-chromosome-linked ichthyosis". Proc. Natl. Acad. Sci. U.S.A. 84 (13): 4519–23. Bibcode:1987PNAS...84.4519B. doi:10.1073/pnas.84.13.4519. PMC 305121. PMID 3474618.
- ^ Bonifas JM, Morley BJ, Oakey RE, Kan YW, Epstein EH (December 1987). "Cloning of a cDNA for steroid sulfatase: frequent occurrence of gene deletions in patients with recessive X chromosome-linked ichthyosis". Proc. Natl. Acad. Sci. U.S.A. 84 (24): 9248–51. Bibcode:1987PNAS...84.9248B. doi:10.1073/pnas.84.24.9248. PMC 299730. PMID 3480541.
- ^ Basler E, Grompe M, Parenti G, Yates J, Ballabio A (March 1992). "Identification of point mutations in the steroid sulfatase gene of three patients with X-linked ichthyosis". Am. J. Hum. Genet. 50 (3): 483–91. PMC 1684279. PMID 1539590.
- ^ a b Ichthyosis, X-Linked at eMedicine: Treatment Section
- ^ DiGiovanna JJ, Robinson-Bostom L (2003). "Ichthyosis: etiology, diagnosis, and management". Am J Clin Dermatol. 4 (2): 81–95. doi:10.2165/00128071-200304020-00002. PMID 12553849. S2CID 243176269.
- ^ Brcic, Lucija; Underwood, Jack FG; Kendall, Kimberley M.; Caseras, Xavier; Kirov, George; Davies, William (2020). "Medical and neurobehavioural phenotypes in carriers of X-linked ichthyosis-associated genetic deletions in the UK Biobank". Journal of Medical Genetics. 57 (10): 692–698. doi:10.1136/jmedgenet-2019-106676. PMC 7525778. PMID 32139392.
- ^ Wren G, Baker E, Underwood JFG, Humby T, Thompson AR, Kirov G, Escott-Price V, Davies W (2022) Characterising heart rhythm abnormalities associated with Xp22.31 deletion Journal of Medical Genetics PMID 36379544 doi:10.1136/jmg-2022-108862 URL:https://jmg.bmj.com/content/early/2022/11/15/jmg-2022-108862.long
- ^ Wren G, Davies W (2024) Cardiac arrhythmia in individuals with steroid sulfatase deficiency (X-linked ichthyosis): candidate anatomical and biochemical pathways Essays in Biochemistry PMID 38571328 doi:10.1042/EBC20230098 URL:https://portlandpress.com/essaysbiochem/article/doi/10.1042/EBC20230098/234273/Cardiac-arrhythmia-in-individuals-with-steroid
- ^ Chatterjee S, Humby T, Davies W (2016) Behavioural and psychiatric phenotypes in men and boys with X-linked ichthyosis: evidence from a worldwide online survey. PLoS One 11(10):e0164417 PMID 27711218 doi:10.1371/journal.pone.0164417 URL: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0164417
- ^ Wren G, Humby T, Thompson AR, Davies W (2022) Mood symptoms, neurodevelopmental traits, and their contributory factors in X-linked ichthyosis, ichthyosis vulgaris and psoriasis. Clinical and Experimental Dermatology PMID 35104372 doi:10.111/ced.15116 URL:https://doi.org/10.1111/ced.15116
- ^ Wren G, Flanagan J, Underwood J, Thompson A, Humby T, Davies W (2024) Memory, mood and associated neuroanatomy in individuals with steroid sulphatase deficiency (X-linked ichthyosis). Genes Brain and Behavior doi:10.1111/gbb.12893 URL:https://onlinelibrary.wiley.com/doi/10.1111/gbb.12893
- ^ Brcic L, Wren GH, Underwood JFG, Kirov G, Davies W (2022) Comorbid medical issues in X-linked ichthyosis. JID Innovations 2(3):100109 PMID 35330591 doi:10.1016/j.xjidi.2022.100109 URL: https://www.jidinnovations.org/article/S2667-0267(22)00016-9/fulltext
- ^ Van Esch H, Hollanders K, Badisco L, et al. (2005). "Deletion of VCX-A due to NAHR plays a major role in the occurrence of mental retardation in patients with X-linked ichthyosis". Hum. Mol. Genet. 14 (13): 1795–803. doi:10.1093/hmg/ddi186. PMID 15888481.
- ^ Wren GH, Davies W (2022) X-linked ichthyosis: New insights into a multi-system disorder Skin Health and Disease PMID 36479267 doi:10.1002/ski2.179 URL:https://onlinelibrary.wiley.com/doi/10.1002/ski2.179
- ^ McGeoghan F, Camera E, Maiellaro M, Menon M, Huang M, Dewan P, Ziaj S, Caley MP, Donaldson M, Enright AJ, O'Toole EA (2023) RNA sequencing and lipidomics uncovers novel pathomechanisms in recessive X-linked ichthyosis Frontiers in Molecular Biosciences 10:1176802 PMID 37363400 doi:10.3389/fmolb.2023.1176802 URL:https://www.frontiersin.org/articles/10.3389/fmolb.2023.1176802/full
- ^ Bradshaw KD, Carr BR (1986). "Placental sulfatase deficiency: maternal and fetal expression of steroid sulfatase deficiency and X-linked ichthyosis". Obstet Gynecol Surv. 41 (7): 401–13. PMID 3531932.
- ^ Cavenagh A, Chatterjee S, Davies W (2019) Behavioural and psychiatric phenotypes in female carriers of genetic mutations associated with X-linked ichthyosis. PLoS One 14(2):e0212330 PMID 30768640 doi:10.1371/journal.pone.0212330 URL:https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0212330
- ^ Lykkesfeldt G, Lykkesfeldt AE, Skakkebaek NE (1984). "Steroid sulphatase in man: a non inactivated X-locus with partial gene dosage compensation". Hum. Genet. 65 (4): 355–7. doi:10.1007/BF00291559. PMID 6582028. S2CID 2625156.
- ^ a b Valdes-Flores M, Kofman-Alfaro SH, Jimenez-Vaca AL, Cuevas-Covarrubias SA (August 2001). "Carrier identification by FISH analysis in isolated cases of X-linked ichthyosis". Am. J. Med. Genet. 102 (2): 146–8. doi:10.1002/ajmg.1450. PMID 11477606.
- ^ a b Cuevas-Covarrubias SA, Kofman-Alfaro S, Orozco Orozco E, Diaz-Zagoya JC (1995). "The biochemical identification of carrier state in mothers of sporadic cases of X-linked recessive ichthyosis". Genet. Couns. 6 (2): 103–7. PMID 7546451.
- ^ Selcer KW, Difrancesca HM, Chandra AB, Li PK (2007). "Immunohistochemical analysis of steroid sulfatase in human tissues". J. Steroid Biochem. Mol. Biol. 105 (1–5): 115–23. doi:10.1016/j.jsbmb.2006.12.105. PMID 17604157. S2CID 22124602.
- ^ Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV (April 2005). "Steroid sulfatase: molecular biology, regulation, and inhibition". Endocr. Rev. 26 (2): 171–202. doi:10.1210/er.2004-0003. PMID 15561802.
- ^ Jöbsis AC, De Groot WP, Tigges AJ, et al. (1980). "X-linked ichthyosis and X-linked placental sulfatase deficiency: a disease entity. Histochemical observations". Am. J. Pathol. 99 (2): 279–89. PMC 1903491. PMID 6929654.
- ^ Elias PM, Crumrine D, Rassner U, et al. (2004). "Basis for abnormal desquamation and permeability barrier dysfunction in RXLI". J. Invest. Dermatol. 122 (2): 314–9. doi:10.1046/j.1523-1747.2003.22258.x. PMID 15009711.
- ^ Bruckner-Tuderman, Leena; Sigg, Christian; Geiger, Jean-Marie; Gilardi, Stefano (1988-04-01). "Acitretin in the Symptomatic Therapy for Severe Recessive X-linked Ichthyosis". Archives of Dermatology. 124 (4): 529–532. doi:10.1001/archderm.1988.01670040031017. ISSN 0003-987X. PMID 2965549.
- ^ Cotellessa C, Cuevas-Covarrubias SA, Valeri P, Fargnoli MC, Peris K (2005). "Topical tazarotene 0.05% versus glycolic acid 70% treatment in X-linked ichthyosis due to extensive deletion of the STS gene". Acta Derm. Venereol. 85 (4): 346–8. doi:10.1080/00015550510026613. PMID 16191859.
- ^ Freiberg RA, Choate KA, Deng H, Alperin ES, Shapiro LJ, Khavari PA (1997). "A model of corrective gene transfer in X-linked ichthyosis". Hum. Mol. Genet. 6 (6): 927–33. doi:10.1093/hmg/6.6.927. PMID 9175741.
- ^ "Press Releases". Timber. Retrieved 2022-04-03.
- ^ Freedberg, et al. (2003). Fitzpatrick's Dermatology in General Medicine. (6th ed.). McGraw-Hill. ISBN 0-07-138076-0.
- ^ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology. (10th ed.). Saunders. ISBN 0-7216-2921-0.
